A scientist from the Bristol Heart Institute has won the British Heart Foundation’s (BHF) annual ‘Reflections of Research’ image competition. Where science and art collide, the competition challenges BHF-funded scientists to showcase their state-of-the-art heart and circulatory disease research through the generation of captivating images.
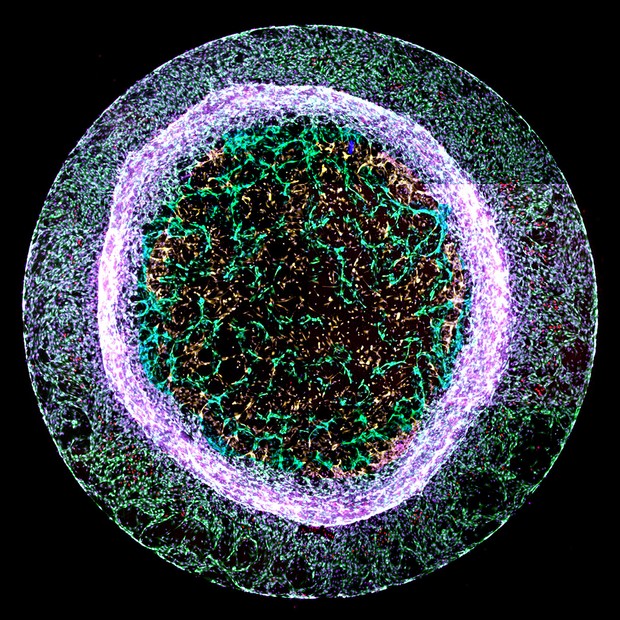
Dr Elisa Avolio’s entry ‘recreating heart blood vessels’ was chosen as this year’s judges’ winner. Although at first glance it appears to resemble a luminous jelly fish, the image shows new blood vessel-like structures – pictured in green in the centre – sprouting from a 3D gel.
Dr Avolio of the Bristol Medical School created the structures using a mixture of two types of heart cells – cardiac endothelial cells, which line the inside of every blood vessel, and pericytes, which ‘hug’ the outside of blood vessels to support the vessel and help it function.
During a heart attack, the arteries that supply blood to the heart are blocked, cutting off blood flow. The area of the heart starved of blood and oxygen dies, and it no longer functions to help the heart pump blood around the body. Dr Avolio is researching ways to encourage the formation of new blood vessels to replace those that have died, to restore blood supply to damaged areas of the heart.
Dr Avolio, a post-doctoral research associate, said:
“It is fantastic to have won this year’s Reflections of Research competition. Each year the entries display such variety in the BHF’s work to support heart and circulatory disease research.
“By recreating models of the heart blood vessels, we can see how the cells in blood vessel walls interact with and talk to other cells. This knowledge, along with understanding what molecules promote or block the formation of blood vessels, could be used in the future to develop new treatments for patients after a heart attack.”
Dr Neil Dufton, Lecturer in Inflammatory Sciences at Queen Mary University of London, was this year’s guest judge. He said:
“All of the images shortlisted in this year’s competition offer a stunning glimpse into the cutting-edge work being carried out by BHF scientists.
“The winning image is truly eye-catching. The chaotic mixture of different cells around the outside contrasts perfectly with a ‘through the looking glass’ moment where we see new and exquisitely detailed blood vessels forming in the centre.”
Dr Charmaine Griffiths, Chief Executive at the British Heart Foundation, was also a competition judge. She added:
“All of this year’s entries beautifully capture aspects of the heart and circulatory system, bringing to life the challenges that BHF scientists work tirelessly to solve.
“The images show how far we’ve come over 60 years of BHF research, and would have been barely imaginable to our founders. I love the winning image not just because of its circular beauty, but also because of the hope it represents for the future of healing damaged hearts.”